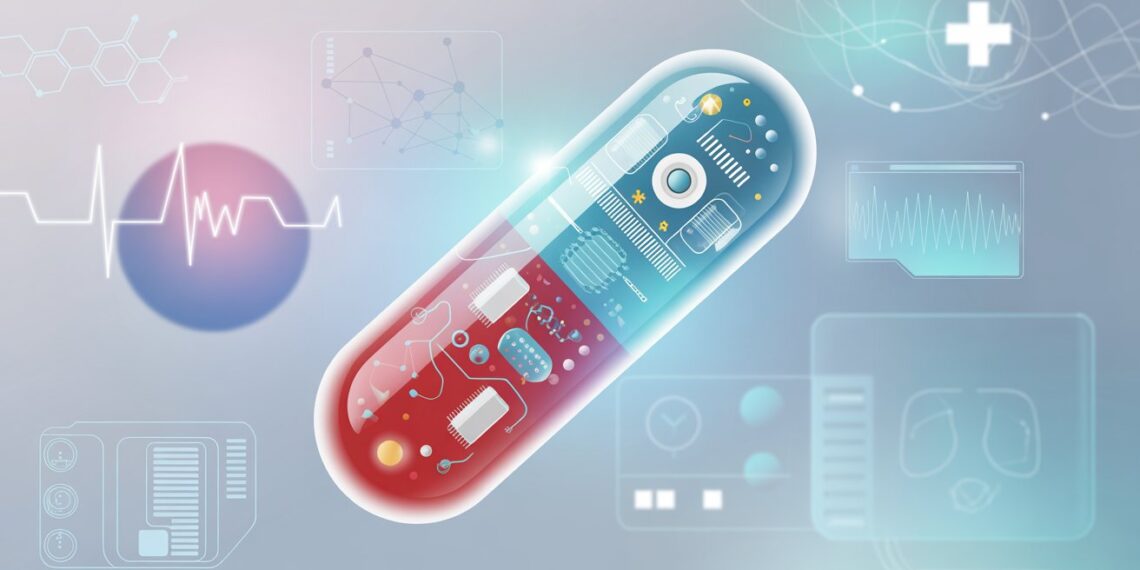
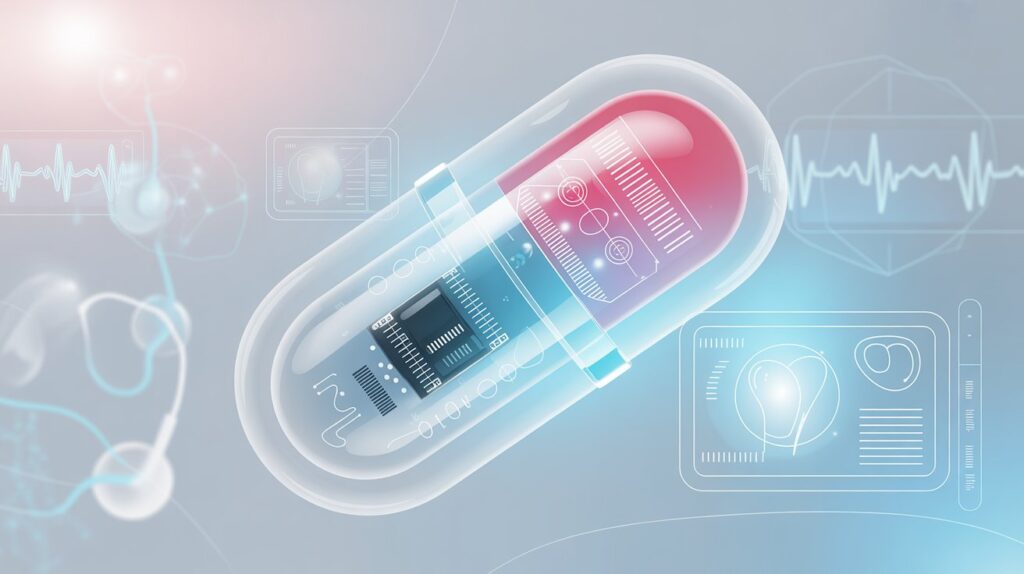
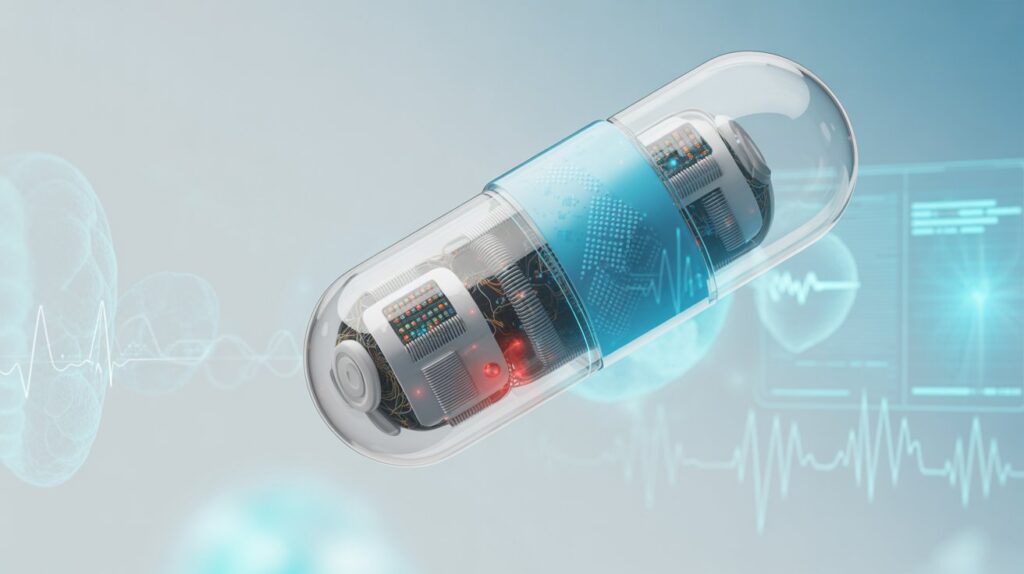
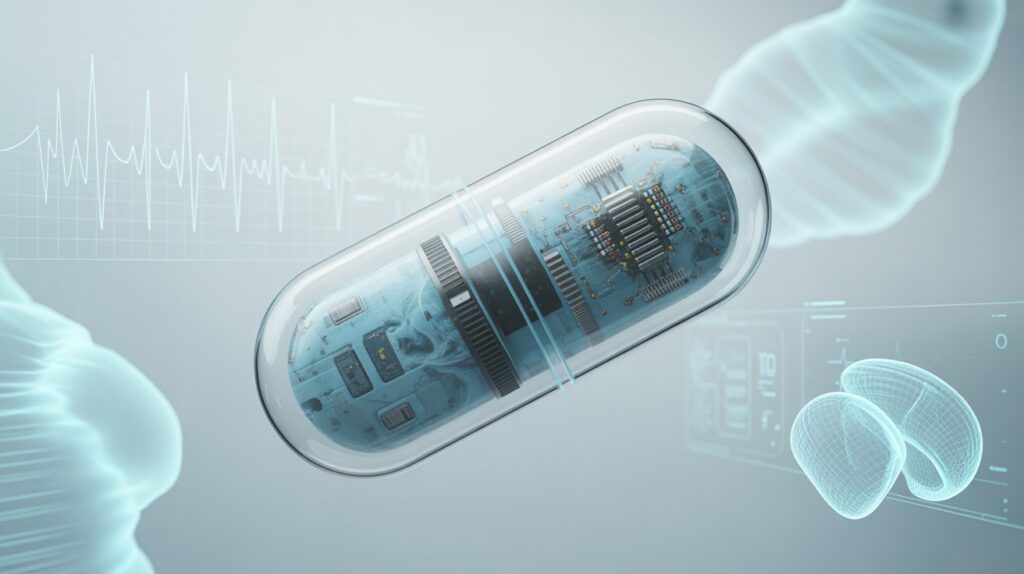
The field of medicine is undergoing a digital revolution. From wearable fitness trackers to implantable sensors, technology is redefining how we monitor health and treat disease. Among the most fascinating breakthroughs of the last decade is the rise of smart pills—tiny, ingestible devices capable of monitoring, diagnosing, and even delivering medication within the human body.
These capsules are not science fiction. They already exist, and their capabilities are expanding rapidly. Smart pills can record internal physiological data, track medication adherence, or deliver drugs to precise locations in the digestive tract. With global healthcare systems under constant pressure to improve patient outcomes, reduce costs, and personalize care, smart pill technology stands at the frontier of digital medicine.
But the question remains: Smart Pills: The Future of Medicine? Will these devices revolutionize healthcare as promised—or are they another overhyped innovation? This article explores how smart pills work, their potential applications, market growth, challenges, ethical concerns, and what lies ahead for this cutting-edge technology.
What Are Smart Pills?
Definition and Core Concept
A smart pill is a miniaturized electronic device, enclosed in a biocompatible capsule, that performs functions beyond traditional drug delivery. Unlike standard oral tablets, smart pills can include sensors, microprocessors, transmitters, and sometimes cameras. Once swallowed, they collect or transmit data about internal bodily functions, drug absorption, or gastrointestinal conditions.
Essentially, they bridge the gap between pharmacology and digital technology, combining therapeutic and diagnostic functions inside a single pill.
How Smart Pills Work
Most smart pills operate through a few essential components:
-
Sensor or Camera Module: Detects physiological parameters—such as pH, temperature, pressure, or visual imagery inside the gastrointestinal tract.
-
Microcontroller Unit: Processes collected data and controls pill operations.
-
Power Source: Either a micro-battery, chemical reaction, or energy harvested from gastric fluids.
-
Wireless Transmitter: Sends data to an external receiver (such as a wearable patch, smartphone, or cloud server).
-
Biocompatible Capsule Shell: Ensures safe ingestion and natural excretion after completing its function.
For example, in capsule endoscopy, a patient swallows a smart pill that travels through the digestive tract, capturing thousands of images of internal tissues. These images are transmitted wirelessly to a receiver, allowing doctors to diagnose ulcers, bleeding, or tumors without invasive procedures.
Evolution of Smart Pill Technology
Early Foundations
The concept of ingestible sensors dates back to the 1980s, but technological constraints made it impractical. Progress in microelectronics, wireless communication, and biocompatible materials during the 2000s reignited research interest.
By 2001, Given Imaging introduced the first capsule endoscope, marking a milestone in noninvasive diagnostics. Over time, pharmaceutical companies and biotech startups began integrating more advanced sensors, cameras, and microprocessors into ingestible capsules.
The Modern Era
Today’s smart pills are far more sophisticated. They can:
-
Measure internal pH levels and temperature
-
Monitor medication adherence (e.g., Proteus Digital Health pill)
-
Deliver targeted doses of drugs at precise locations
-
Capture video imaging for the entire GI tract
-
Analyze biomarkers to detect early signs of disease
Recent advances even include AI-assisted analysis, enabling predictive diagnostics and early intervention before symptoms become critical.
Applications of Smart Pills in Modern Healthcare
1. Diagnostic Imaging
Capsule endoscopy remains the most established application. Instead of traditional endoscopy, which requires inserting tubes through the throat or rectum, patients swallow a camera capsule. It travels naturally through the GI tract, capturing high-resolution images that help detect:
-
Gastrointestinal bleeding
-
Crohn’s disease
-
Ulcers
-
Tumors
-
Polyps
This approach offers comfort, convenience, and more complete visualization of the small intestine—an area conventional endoscopy often misses.
2. Drug Delivery and Controlled Release
Smart pills can release medication at targeted sites, such as the small intestine or colon, improving drug efficacy while minimizing side effects. This is particularly beneficial for conditions like ulcerative colitis or Crohn’s disease, where localized treatment reduces systemic exposure.
Some research prototypes are developing trigger-based systems, where sensors detect environmental cues (like pH or temperature) and release drugs only when conditions indicate therapeutic need.
3. Medication Adherence Monitoring
One of the most pressing issues in healthcare is patient nonadherence—failure to take medication as prescribed. According to the World Health Organization, nonadherence contributes to nearly 50% of treatment failures and billions of dollars in preventable healthcare costs each year.
Smart pills equipped with ingestible sensors can communicate with external patches or apps to confirm ingestion. Physicians and caregivers receive real-time data, improving accountability and ensuring consistent treatment—especially for chronic diseases like hypertension, diabetes, and schizophrenia.
4. Physiological and Biomarker Monitoring
Some advanced capsules can monitor internal biomarkers—such as glucose levels, temperature, or gastric pressure—providing data for personalized treatment. These applications can transform chronic disease management, especially for patients with diabetes or gastrointestinal disorders.
5. Early Disease Detection
Integrating biosensors with AI can enable predictive diagnostics. For instance, continuous internal monitoring may detect inflammation, infection, or micro-bleeding long before symptoms surface. This allows for preventive intervention rather than reactive treatment.
Market Trends and Industry Outlook
The global smart pill market has grown significantly, driven by technological innovation, increasing chronic disease prevalence, and demand for minimally invasive diagnostics.
-
According to DelveInsight (2024), the smart pill market is projected to grow at a compound annual growth rate (CAGR) of around 11.3% between 2024 and 2030.
-
The AmericanHHM report (2023) attributes this growth to rising adoption in gastrointestinal diagnostics, advancements in nanotechnology, and improved wireless communication standards.
-
North America currently dominates the market, followed by Europe and Asia-Pacific. However, emerging economies are expected to see the fastest adoption due to growing healthcare investments.
Key players include Medtronic, CapsoVision, Proteus Digital Health, Olympus Corporation, and Check-Cap Ltd.
Technological Drivers Behind Smart Pills
1. Miniaturization of Components
Advances in microelectromechanical systems (MEMS) have made it possible to embed sensors, processors, and batteries into millimeter-scale capsules.
2. Biocompatible Materials
Improved polymers and coatings ensure that smart pills safely withstand stomach acids and intestinal fluids without degrading prematurely.
3. Wireless and IoT Integration
5G, Bluetooth Low Energy, and near-field communication technologies allow seamless data transfer to smartphones and cloud systems for real-time monitoring.
4. Artificial Intelligence and Machine Learning
AI helps interpret massive data streams from ingestible sensors—detecting patterns, predicting disease risk, and automating diagnostic workflows.
5. Power and Energy Innovations
New research explores using biochemical energy sources—such as stomach acid—as power supplies, eliminating traditional batteries and extending capsule lifespan.
Ethical, Privacy, and Safety Considerations
While smart pills offer tremendous promise, their adoption raises complex ethical and regulatory questions.
1. Data Privacy and Ownership
Smart pills generate intimate biological data. Who owns this information—the patient, doctor, or manufacturer? Protecting personal health data from misuse or unauthorized access is a major concern, especially as it’s transmitted wirelessly.
2. Informed Consent and Autonomy
Patients must fully understand what data is collected and how it’s used. For example, digital adherence pills can track when medication is ingested—potentially crossing ethical lines if used coercively in mental health or correctional settings.
3. Safety and Biocompatibility
Regulators require extensive testing to ensure capsules dissolve or pass safely. Malfunctioning devices could pose health risks, especially in patients with strictures or obstructions in the digestive tract.
4. Psychological Impact
Knowing one’s body is under constant internal surveillance may create anxiety or distrust among patients. A balance must be maintained between monitoring and personal comfort.
5. Accessibility and Equity
As with many advanced technologies, cost is a barrier. Without affordable access, smart pills risk widening healthcare disparities between developed and developing regions.
Regulatory Landscape
Smart pills fall under medical device and pharmaceutical regulations, often creating hybrid approval challenges.
-
In the United States, the Food and Drug Administration (FDA) classifies smart pills as combination products—both drug and device—requiring rigorous evaluation of safety, efficacy, and data security.
-
The European Medicines Agency (EMA) and Japan’s PMDA follow similar frameworks, though approval processes vary.
-
The Proteus Digital Health pill became the first FDA-approved ingestible sensor system in 2017, paving the way for future entrants.
Clearer international standards are needed to accelerate innovation while maintaining patient safety.
Comparison with Other Health Technologies
Feature |
Smart Pills |
Wearables |
Implantables |
|---|---|---|---|
Data Source |
Internal (GI tract, stomach) |
External (skin, wrist) |
Internal (implanted under skin) |
Invasiveness |
Noninvasive ingestion |
Noninvasive |
Minimally invasive |
Accuracy |
High (direct physiological data) |
Moderate (surface-level) |
Very high (continuous monitoring) |
Use Duration |
Short-term (hours to days) |
Continuous |
Long-term |
Main Use |
Diagnostics, drug delivery |
Fitness, chronic monitoring |
Continuous health tracking |
This comparison highlights that smart pills occupy a unique niche—they are less invasive than implants but provide more detailed data than wearables.
Challenges to Widespread Adoption
1. Cost and Reimbursement
Many health systems have yet to include smart pill procedures under insurance coverage. Without reimbursement pathways, adoption remains limited to research or high-end private healthcare.
2. Technological Limitations
Battery life, data accuracy, and potential for interference remain technical challenges. Capsules must balance functionality with safety and miniaturization.
3. Public Acceptance
Adoption depends not just on medical efficacy but also on trust. People must feel comfortable swallowing digital devices.
4. Clinical Validation
Large-scale clinical trials are required to prove long-term benefits. Only a handful of products currently have FDA or CE approval.
Future Directions and Research Opportunities
Personalized Medicine
Smart pills can transform treatment personalization by enabling data-driven dosage adjustments and real-time monitoring. Combined with genomic data and AI, they could tailor therapies for each individual’s biology.
Integration with Telemedicine
As remote healthcare expands, ingestible sensors can act as internal “data reporters,” transmitting physiological metrics to physicians anywhere in the world.
Nanotechnology and Bio-Hybrid Designs
Researchers are exploring nano-smart pills that can target specific cells or tissues. Future capsules may even incorporate biodegradable microrobots capable of performing minor interventions, such as unclogging arteries or repairing tissue damage.
Preventive Healthcare
Instead of diagnosing illness after it develops, smart pills could continuously monitor internal markers, identifying early signs of disease and triggering alerts long before symptoms appear.
Expert and Industry Perspectives
-
Dr. Anthony Wong, biomedical engineer at MIT, predicts that “smart pills will be as common as wearables within two decades, driven by better energy harvesting and AI analytics.”
-
Market analysts foresee strong adoption in gastrointestinal care, oncology, and chronic disease management, particularly where patient adherence is critical.
-
Pharmaceutical firms are partnering with tech companies to develop hybrid ecosystems combining smart drugs, sensors, and cloud platforms—blurring the line between medicine and digital technology.
Potential Societal Impact
-
Reduced Hospital Visits: Noninvasive monitoring could lower diagnostic procedure demand.
-
Better Treatment Outcomes: Continuous adherence tracking ensures medications are taken correctly.
-
Cost Efficiency: Preventive insights reduce long-term healthcare costs.
-
Patient Empowerment: Access to personal health data enables informed decisions.
-
New Ethical Frontiers: Data ownership debates could shape digital rights legislation in healthcare.
Smart Pills: The Future of Medicine?
Returning to the core question—Smart Pills: The Future of Medicine?—the answer is cautiously optimistic. Smart pills are already transforming diagnostics and treatment in niche areas, and their future potential is vast. However, realizing that future depends on solving issues of cost, regulation, ethics, and patient acceptance.
They may not replace traditional medicine, but they will complement and enhance it, creating a healthcare ecosystem that is smarter, more responsive, and increasingly personalized.
Frequently Asked Questions (FAQs)
1. Are smart pills safe to swallow?
Yes. Approved smart pills are made from biocompatible materials that pass naturally through the digestive system. Each undergoes extensive clinical testing for safety and tolerability.
2. What are the most common uses of smart pills today?
Current uses include gastrointestinal imaging, medication adherence monitoring, and site-specific drug delivery.
3. Can smart pills replace endoscopy?
In many cases, capsule endoscopy can substitute traditional endoscopy for small intestine imaging. However, for biopsies or surgical interventions, standard endoscopy is still required.
4. Who manufactures smart pills?
Major companies include Medtronic, CapsoVision, Olympus, and Check-Cap. Startups like etectRx and Proteus Digital Health have pioneered digital adherence capsules.
5. Do smart pills transmit personal data?
Yes, they transmit specific health metrics wirelessly, usually to secure medical servers. Patients should ensure their provider follows strict data protection standards.
6. When will smart pills become mainstream?
Experts predict widespread use in diagnostics within the next 5–10 years, especially as costs decrease and regulations adapt.
Conclusion
Smart pills represent one of the most exciting frontiers in modern healthcare—merging biotechnology, microelectronics, and artificial intelligence to create truly digital medicine.
Their promise lies in enabling noninvasive diagnostics, precise drug delivery, and continuous internal monitoring—all of which can save lives, cut costs, and redefine preventive care. But to fulfill that promise, the industry must address real barriers: affordability, regulation, ethical governance, and patient trust.
The coming decade will determine whether smart pills become a routine part of healthcare or remain a niche innovation. For now, one thing is clear—they symbolize the convergence of medicine and technology in its most intimate form, bringing us closer to a future where treatment is as intelligent as the diseases it fights.